Health Advice
/Health

'Dreamers' can enroll in ACA plans this year -- but a court challenge could get in the way
When open enrollment for the Affordable Care Act, or Obamacare, starts nationwide this week, a group that had previously been barred from signing up will be eligible for the first time: The “Dreamers.” That’s the name given to children brought to the United States without immigration paperwork who have since qualified for the Deferred ...Read more

The 4 types of systemic therapy for breast cancer
Treatment for breast cancer can come in many forms. In addition to surgery to remove cancerous tissue and radiation therapy, breast cancer also is commonly treated with drugs that are taken orally or intravenously, as an infusion into a vein.
Drugs that treat cancer also are called systemic therapies. Types of systemic therapies for breast ...Read more

Mayo Clinic Minute: Stroke treatment
Each year, an estimated 15 million people around the globe experience stroke, according to the World Health Organization, with one-third resulting in death. That's why immediate stroke treatment is crucial.
When the symptoms of stroke present, calling 911 and seeking care immediately is the most crucial step to prevent disability or death.
“...Read more

Bridging the digital divide to help rural smokers quit
Rural adults are more likely to smoke than their urban counterparts. Enhancing digital literacy and improving access to the internet and digital devices may make it easier for rural smokers to quit. These are the findings of a randomized, controlled pilot clinical trial Mayo Clinic researchers published in Nature Communications Medicine.
...Read more
Overdose deaths are rising among Black and Indigenous Americans
The recent decline in overdose deaths hides a tremendous disparity by race: Deaths have fallen only among white people while continuing to rise among people of color, according to a new Stateline analysis of federal data.
Health experts in nonwhite communities say they’re finding strategies that work in their areas, but that they still ...Read more

Mayo Clinic Q and A: What is cardiac amyloidosis?
DEAR MAYO CLINIC: My dad was diagnosed with cardiac amyloidosis shortly after his 70th birthday. It's difficult to pronounce let alone understand. What is cardiac amyloidosis? Am I at risk if it is genetic?
ANSWER: Amyloidosis is a rare condition defined by the abnormal production of proteins that bind together to form amyloid proteins. These ...Read more
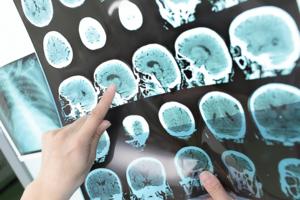
What new guidelines say to do to prevent a stroke
Hundreds of thousands of people in the U.S. have a first stroke each year. Newly updated recommendations spell out methods for changing that.
The new guidelines from the American Heart Association and American Stroke Association, published in the journal Stroke, aim to prevent stroke throughout a person's life via preventive care and healthy ...Read more

Mothering over meds: Docs say common treatment for opioid-exposed babies isn't necessary
On learning last year she was pregnant with her second child, Cailyn Morreale was overcome with fear and trepidation.
“I was so scared,” said Morreale, a resident of the small western North Carolina town of Mars Hill. In that moment, her joy about being pregnant was eclipsed by fear she would have to stop taking buprenorphine, a drug used ...Read more

In 'concerning development,' officials say H5N1 bird flu has infected a pig in Oregon
Oregon state and federal officials confirmed Wednesday that H5N1 bird flu was found in a pig living Crook County — the first such swine infection reported in the current outbreak.
The strain of bird flu virus in the pig is slightly different than the one that has been plaguing dairy cows in California and other states, which is known as B3.13...Read more

How to choose the healthiest salad dressing
Salad dressings are an important part of the taste and nutrition of your salad. But with countless varieties of salad dressings on store shelves, picking out a healthy one can feel overwhelming. Here’s how to choose a healthy salad dressing, including what ingredients to look for and avoid, and nutrition stats to be aware of.
What makes salad...Read more

Have you exfoliated lately?
Social media has a way of making the ho-hum seem fresh and novel. Case in point: exfoliation, the process of removing dead cells from the skin’s outer layer. Anyone scrolling through TikTok might be convinced this longtime skin care approach can transform something old — let’s say our aging epidermis — into like-new skin.
But a Harvard ...Read more

Mayo Clinic Q&A: Is snacking for meals acceptable for good health?
DEAR MAYO CLINIC: During warm weather months, I find myself eating less formal meals and, instead, reaching for more snacks. I also tend to exercise less when it’s hot outside. Do you have advice on how to ensure I’m snacking healthfully as I realize the potential to overeat and not be as active?
ANSWER: Regardless of the time of year, ...Read more

PBM math: Big chains are paid $23.55 to fill a blood pressure prescription. Small drugstores get $1.51
CUTHBERT, Ga. — While customers at Adams Family Pharmacy picked up their prescriptions on a hot summer day, some stopped in for coffee, ice cream, homemade cake, or cookies.
It wasn’t a bake sale, but the sweets bring extra revenue as pharmacist and co-owner Nikki Bryant works to achieve profitability at her business on the town square.
...Read more
Some Americans need more than one COVID booster this season, CDC says
Cold and flu season is upon us, and with it comes a new round of COVID-19 booster shots.
The new 2024-2025 shots were recommended by the Centers for Disease Control and Prevention in June, approved and authorized by the Food and Drug Administration in August and rolled out to doctors offices and pharmacies in September.
Expecting an increase ...Read more
For reproductive health workers, a big change since the Dobbs ruling
MEMPHIS, Tenn. —These days, half of what was the first nonprofit clinic in the nation to house a birthing center and provide abortions is empty.
The clinic is CHOICES – Memphis Center for Reproductive Health and it opened in 1974, in the aftermath of the Supreme Court’s Roe v. Wade ruling.
But now its abortion patient clinical rooms and ...Read more

Patients are relying on Lyft, Uber to travel far distances to medical care
When Lyft driver Tramaine Carr transports seniors and sick patients to hospitals in Atlanta, she feels like both a friend and a social worker.
“When the ride is an hour or an hour and a half of mostly freeway driving, people tend to tell you what they’re going through,” she said.
Drivers such as Carr have become a critical part of the ...Read more

Can I get bird flu from eating eggs? Drinking milk? We asked a California disease expert
SACRAMENTO, Calif. -- As poultry farms and dairies across California battle bird flu outbreaks, some are wondering if their food is safe to eat.
Since highly pathogenic avian influenza surfaced in the United States in January 2022, the virus has been detected in wild birds and domestic poultry, according to the federal Centers for Disease ...Read more
New COVID vaccine booster: Why you should wait
Yes, older Americans should get yet another COVID shot – but if you have already gotten the latest version, there’s no rush.
The Centers for Disease Control and Prevention last week said that people 65 and older or who are immunocompromised need a second dose of the new vaccine released in September.
But, you should wait six months after ...Read more

How to protect trick-or-treaters from cannabis edibles, allergens, and more this Halloween
PHILADELPHIA — It's a standard safety measure that parents regularly conduct after their children finish trick-or-treating: checking the kids' Reese's Pieces and Mars bars and Nerds Ropes to make sure none have been opened or tampered with.
But some Halloween dangers are closer to home — and more likely to occur than the threat of someone ...Read more
Mayo Clinic Minute: 3 tips to avoid Halloween hand injuries
There are plenty of frights to go around on Halloween, but a hand injury probably isn't one you'd expect.
"Interestingly, it's the fourth busiest holiday for hand injuries," says Dr. Sanj Kakar, a Mayo Clinic orthopedic hand and wrist surgeon.
Dr. Kakar says almost one-third of those Halloween hand injuries are among kids ages 10 to 14. And ...Read more
Popular Stories
- Bridging the digital divide to help rural smokers quit
- In 'concerning development,' officials say H5N1 bird flu has infected a pig in Oregon
- How to choose the healthiest salad dressing
- What new guidelines say to do to prevent a stroke
- Mayo Clinic Q&A: Is snacking for meals acceptable for good health?








