Current News
/ArcaMax

In election year, US Rep. Mary Peltola walks a complicated path on fisheries and oil drilling
ANCHORAGE, Alaska — U.S. Rep. Mary Peltola of Alaska this week unexpectedly pulled her support for a Republican-led bill that seeks to pave the way for more oil drilling in the state, opening her up to criticism from opponents who claim she's supporting a pro-Biden agenda.
It was the latest in a series of moves by the Democrat that has led to...Read more

Cinco de Mayo: You might be surprised how long it's been celebrated in the U.S.
Cinco de Mayo celebrates the Mexican victory over the French at the Battle of Puebla, which took place on May 5, 1862.
It is not observed as a national holiday in Mexico, but schools in Puebla, where the battle took place, are closed for the day.
Mexico’s Independence Day is Sept. 16.
A report published by the UCLA Center for the Study of ...Read more

New Jersey family repatriates rare artifacts to Philadelphia's Mexican Consulate
In a repatriation ceremony at the Consulate of Mexico in Philadelphia on Wednesday, a collection of culturally significant archaeological pieces — some going back almost 2,500 years — was returned to the Mexican people by the family of a private collector.
"The 22 pieces before you today are recognized as movable archaeological monuments .....Read more

Before mob attack, UCLA police chief was ordered to create security plan but didn't, sources say
LOS ANGELES — On the morning before a mob attacked a pro-Palestinian student encampment at UCLA, campus Police Chief John Thomas assured university leadership that he could mobilize law enforcement “in minutes” — a miscalculation from the three hours it took to actually bring in enough officers to quell the violence, according to three ...Read more

While a few other universities reach compromises with protesters, why can't Penn?
PHILADELPHIA — At Rutgers University-New Brunswick, a three-day-old pro-Palestinian encampment came down peacefully Thursday after successful negotiations between the administration and protesters.
Brown University, an Ivy League institution like the University of Pennsylvania, and Northwestern University had similar success earlier this week...Read more

Longtime Trump loyalist Hope Hicks in tears after potentially damaging testimony in hush money trial
NEW YORK — Donald Trump’s loyal former White House press secretary Hope Hicks testified at his Manhattan trial Friday, breaking down after divulging potentially damaging testimony for the former president regarding the motivations behind his fixer’s hush money payoff to porn star Stormy Daniels.
Trump’s former White House Communications...Read more

Trump hush-money trial: Key takeaways from week two of testimony
NEW YORK — The second week of testimony in Donald Trump’s hush-money criminal trial gave jurors first-hand accounts of negotiations for payments to two women and how the former president’s 2016 campaign reacted when news of their alleged affairs leaked out anyway.
Hope Hicks, who was press secretary for Trump’s campaign and became a top...Read more

'Extraordinary honor': Biden gives Presidential Medal of Freedom to Pelosi, Clyburn, Kerry and others
https://www.gettyimages.com/detail/news-photo/president-joe-biden-awards-the-medal-of-freedom-to-u-s-rep-news-photo/2151325031?adppopup=true
and
https://www.gettyimages.com/detail/news-photo/president-joe-biden-awards-the-medal-of-freedom-to-former-news-photo/2151331538?adppopup=true
WASHINGTON — President Joe Biden on Friday awarded the ...Read more

NYC politicians demand Mayor Adams discipline NYPD Chief John Chell over 'dangerous' tweets
NEW YORK — More than three dozen local elected officials are calling on Mayor Eric Adams to discipline New York Police Department Chief John Chell over “illegal” and “dangerous” social media attacks that the police honcho has leveled against lawmakers, journalists and others in recent months.
In a Friday letter to Adams exclusively ...Read more
News briefs
Opal Lee, grandmother of Juneteenth, to be honored with Presidential Medal of Freedom
Opal Lee, known as the “grandmother of Juneteenth,” will be awarded the Presidential Medal of Freedom, the nation’s highest civilian honor, White House officials announced.
Lee, 97, is among 19 recipients of the honor the White House said is presented ...Read more
18 arrested in Nevada in operation targeting online child sex predators
LAS VEGAS — A multi-agency operation targeting online child sex predators led to 18 arrests in Henderson last weekend, according to authorities.
The joint operation took place on April 26 and 27, the Metropolitan Police Department said in a news release.
The operation included detectives and agents from the Internet Crimes Against Children ...Read more

Student posted airsoft rifle photo in threat aimed at Kentucky high school
LEXINGTON, Ky. — A Fayette County student on Friday posted a threat that included an airsoft rifle and mentioned Tates Creek High School, Principal Kristy Field said in a letter to parents.
“This morning we became aware of a social media post made by a student on Snapchat that began circulating among students and staff,” Field said. “...Read more

California roads damaged by storms could get help with Gov. Newsom's emergency declaration
LOS ANGELES — Gov. Gavin Newsom proclaimed a state of emergency Friday to help fund badly needed repairs of roads battered during this year's storms, including scenic Topanga Canyon Boulevard that was blocked by millions of tons of debris.
The governor's action comes two weeks after California Department of Transportation officials said the ...Read more

Three friends drove from California to Mexico for a surfing trip. Then they disappeared
MEXICO CITY — Last month, two brothers and one of their friends crossed from the United States into Mexico to explore Baja California's famous surf breaks. Pictures posted online by one of the brothers, Callum Robinson, 33, show the men gazing out at the ocean with coffee, enjoying street tacos and relaxing with beers on a roof deck.
After ...Read more

Longtime Trump aide Hope Hicks breaks down during hush money trial testimony
NEW YORK — Donald Trump’s loyal former White House aide Hope Hicks testified at his Manhattan trial Friday, tearing up when questioning turned to what Trump knew about his fixer’s hush money payoff to porn star Stormy Daniels.
Trump’s former White House communications director and campaign spokeswoman, once considered among the former ...Read more

Jailed students, a canceled commencement, angry parents: USC's Carol Folt takes on critics
LOS ANGELES — When University of Southern California trustees selected Carol Folt as their next president, they gave her one of the most challenging mandates in American higher education: Restore trust in a university diminished by scandals.
She replaced key administrators, brokered a $1 billion settlement with alumnae victimized by a ...Read more
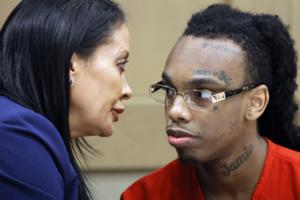
Judge's ruling puts defense 'back in the saddle' on YNW Melly murder case
FORT LAUDERDALE, Fla. — Defense lawyers in the YNW Melly murder case are getting back to work, at least behind the scenes, though it will still be months before a second jury hears the bloody details.
Broward Circuit Judge John J. Murphy issued a ruling this week allowing background work, such as sworn witness interviews, to continue while ...Read more
Serial Chicago con man gets 9 years in federal prison for 'extraordinarily large amount of offenses'
CHICAGO — Serial Chicago con man Joey Cipolla was sentenced to nine years in federal prison Friday for what the judge called “an extraordinarily large amount” of schemes to cheat people out of money.
In handing down the 108-month sentence, U.S. District Judge Matthew Kennelly noted that Cipolla had spent time in state prison for similar ...Read more

Bill awaiting DeSantis' OK would end years of renewable energy policies
TALLAHASSEE, Fla. — A bill sitting on Gov. Ron DeSantis’ desk would end the state’s support of renewable and clean energy and keep Florida reliant on fossil fuels, critics say.
If signed, the law would reverse 16 years of state policy, finishing the work started by former Gov. Rick Scott and undoing Gov. Charlie Crist’s signature piece ...Read more

Police report no serious injuries. But scenes from inside UCLA camp, protesters tell a different story
LOS ANGELES — It was a request that police had made repeatedly: Stop throwing things at officers. But as pro-Palestinian demonstrators made their last stand Thursday morning in defense of the encampment they’d occupied at UCLA for the better part of a week, some protesters did not comply.
After another piece of wood or a plastic water ...Read more
Popular Stories
- Three friends drove from California to Mexico for a surfing trip. Then they disappeared
- United Methodists look to move forward after anti-LGBTQ language is removed
- Trailblazing former Sen. Elizabeth Dole to receive Presidential Medal of Freedom
- UGA professors want protesting students' suspensions lifted
- Antisemitic fliers found littering neighborhood near Emory





