Current News
/ArcaMax
News briefs
Marjorie Taylor Greene faces GOP backlash after failed bid to oust speaker
WASHINGTON — A throng of media crowded around U.S. Rep. Marjorie Taylor Greene on Wednesday evening on the steps of the U.S. Capitol, straining to hear her angry reaction after a bipartisan coalition of House members dismissed her efforts to oust Mike Johnson as House ...Read more
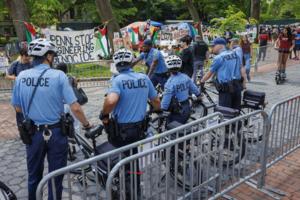
Gov. Josh Shapiro calls for Penn to disband pro-Palestinian encampment as 6 students are placed on leave
Citing increasing unlawfulness, Gov. Josh Shapiro on Thursday called on the University of Pennsylvania to disband a pro-Palestinian encampment that has spent two weeks on the Ivy League campus.
“Over the last 24 hours ... the situation has gotten even more unstable and out of control,” Shapiro said during an event in Westmoreland County. �...Read more

NY state prison system breaks law by sending disabled inmates to solitary: lawsuit
NEW YORK — The New York State prison system continues to send inmates with mental illness disabilities to solitary confinement, despite a 2022 state law banning them from doing so, according to a new class action lawsuit.
The suit, filed in Brooklyn Supreme Court, is accusing the state of placing inmates with myriad physical and mental ...Read more

Famed Ansel Adams photos of Yosemite, Golden Gate to be featured on new US stamps
For more than 150 years, visitors have taken hundreds of millions of photographs of Yosemite National Park.
But many of the park’s most iconic images — timeless, internationally famous shots of Half Dome, Tunnel View, Mirror Lake and other wonders that strikingly depict America’s natural heritage — were made by Bay Area native Ansel ...Read more

Crews prepare explosives for controlled demolition of Key Bridge section atop Dali
BALTIMORE — Salvage crews have been preparing explosive charges that will be used in a controlled demolition of the section of the Key Bridge that landed atop the Dali container ship, officials said Thursday, which will allow the vessel to eventually return to the Port of Baltimore.
The Key Bridge Unified Command, which is heading recovery ...Read more

Business owner dumps pollutants into Ohio river, killing 40,000 fish, feds say
A business owner has pleaded guilty to dumping thousands of gallons of hazardous substances into an Ohio river, killing more than 40,000 fish, officials said.
“There’s a right way and a wrong way to do business,” Ohio Attorney General Dave Yost said in a May 7 news release. “When your business pollutes Ohio’s natural resources, you ...Read more

Alaska House adopts capital budget in near-unanimous vote
JUNEAU, Alaska — The Alaska House adopted the capital budget in a 39-1 vote Wednesday, displaying a rare moment of cohesion between the Republican-controlled majority and the more progressive minority.
Rep. Bryce Edgmon, a Dillingham independent who oversaw the capital budget in the House as co-chair of the House Finance Committee, said the ...Read more

Chinese billionaire with ties to Mayor Adams sentenced to deportation, time served
NEW YORK — Hui Qin, the Chinese billionaire who admitted in March to making straw donations to political campaigns — including Mayor Eric Adams’ — was sentenced Thursday to seven months in prison and removal from the U.S.
Brooklyn federal prosecutor Breon Peace, who handled the case, called the sentencing “a lesson in American civics ...Read more

Maryland early voting ends quietly ahead of Tuesday's primary election
The trickle of voters who kicked off early in-person voting last week seemed to continue Thursday as the last day to cast a ballot in person before Tuesday’s primary drew to a close.
By Thursday afternoon, almost 150,000 Marylanders had cast early in-person votes, compared to the 178,000 who voted early in the 2022 primary, according to Jared...Read more

$187,000 marijuana top job opens up to nationwide search in Massachusetts
BOSTON — The continued success of the Bay State’s legal weed business will need a “tough, strategic thinker” to oversee day-to-day administration of the agency in charge of the $7 billion pot industry, according to the Cannabis Control Commission.
The CCC has begun a nationwide job search for its second Executive Director, the person ...Read more

Should you be worried about bird flu? Here are 5 things to know about the virus
As a new virus takes center stage at the heart of a global outbreak, it’s easy to get flashbacks of March 2020.
Now more than four years after the world was rocked by a pandemic, H5N1, or avian or bird flu, has exploded in bird and livestock populations, and at least one human case has been confirmed by health officials.
This isn’t the ...Read more

NYPD brass refuse to answer Council questions on controversial social media posts during tense hearing
NEW YORK — After two months of speaking their minds on social media, NYPD officials were tight-lipped about their controversial posts at a City Council hearing Thursday as two department executives responsible for most of the tweets were no-shows.
NYPD Chief of Patrol John Chell and Commissioner of Operations Kaz Daughtry skipped the morning ...Read more

Ukraine to receive US-made mobile rocket systems paid by Germany
Ukraine will receive three HIMARS mobile rocket systems from U.S. stocks with Germany footing the bill, German Defense Minister Boris Pistorius said Thursday.
Pistorius, who revealed the plan after talks with U.S. Defense Secretary Lloyd Austin at the Pentagon, said the idea emerged during the extended wait for $61 billion in U.S. aid to ...Read more

New clues revealed in 'senseless' killing of 19 wild burros, California officials say
Land management officials revealed new clues that will hopefully lead to a breakthrough in the killing of 19 burros along a California highway in 2019.
The Bureau of Land Management also offered another $10,000 on top of contributions from several animal rights groups, bringing the total to $36,000 for information that leads to justice in the ...Read more
Bloods 'godfather' nicknamed 'Chucky' gets 23 years in federal prison
A violent Brooklyn Bloods “godfather” leader who once blasted a disloyal gang member’s leg off was sentenced Thursday to 23 years in federal prison.
Quandell “Chucky” Smothers, 33, was such a terrifying presence in East New York that the man whose leg he shot off lied about it on the witness stand, the feds said — despite ...Read more

Boston Chamber chief urges City Council to rein in Mayor Wu's spending amid economic uncertainty
BOSTON — The head of the Greater Boston Chamber of Commerce criticized the mayor’s decision to raise the city budget by 8% amid economic uncertainty while urging the City Council to practice “fiscal discipline” by making cuts to her spending proposal.
In a letter addressed to Councilors Brian Worrell and Enrique Pepén, chair and vice ...Read more
Black airman shot to death by Florida deputies who blitzed wrong apartment: attorneys
A Black U.S. Air Force airman was on the phone with his girlfriend one afternoon when he heard someone pounding on the door of his apartment in the Florida Panhandle.
After looking through the peephole and noticing that it was covered, he grabbed his gun — and sheriff’s deputies stormed inside. His girlfriend, attorneys say, listened in as ...Read more

White House to push cybersecurity standards on hospitals after Change Healthcare breach
WASHINGTON — The Biden administration intends to require hospitals to meet minimum cybersecurity standards after a single hack exposed the data of 100 million Americans, according to a senior U.S. cybersecurity official.
“We look to putting in place minimum cybersecurity standards for hospitals in the near term,” Anne Neuberger, deputy ...Read more

Prince Harry invited royal family to celebrate 10th anniversary of Invictus Games -- all skipped
Prince Harry reportedly invited King Charles, Prince William and Kate Middleton to attend a milestone service for the 10th anniversary of his Invictus Games in London this week, though none of them attended.
The 39-year-old Duke of Sussex invited the trio to join him at Wednesday’s Service of Thanksgiving at St. Paul’s Cathedral, per People...Read more

Deadly brain disease found in 2 California deer
LOS ANGELES — State officials reported the presence of deadly chronic wasting disease in two wild California deer earlier this week. This is the first time the disease, which has plagued other areas of the nation for years, has appeared in the state’s deer or elk population.
The positive samples came from a deer that died of unknown causes ...Read more
Popular Stories
- Stormy Daniels fends off accusations she made up story of sex tryst with Trump at hush money trial
- New COVID 'FLiRT' variants are spreading nationwide. Chicago health experts urge up to date vaccination
- After mass shooting, Miami-area's nightlife may see changes. How late could bars stay open?
- Supreme Court rules innocent owners of seized cars are not entitled to immediate hearing
- This city, very carefully, calls for end to Mideast 'hostilities.' It's a first for Miami-Dade





