Current News
/ArcaMax

Emerson College protest arrests divides along public safety vs political lines
The arrest of 108 at Emerson College in the pre-dawn hours had politicians taking shots at the police who warned the students pitching tents in a public way gave them no choice.
Social media video of the police sweep shows protesters being forcibly removed from the Boylston Place location around 2 a.m. Thursday, with some refusing to move in a ...Read more

Kim Jong Un tests new rockets to strike Seoul and perhaps sell to Putin
Leader Kim Jong Un oversaw tests of a new North Korean rocket system that could bolster his ability to attack Seoul and it may become a weapon he attempts to sell to Russia for use in its assault on Ukraine.
Kim watched the test-fire of a 240 millimeter multiple rocket launcher with shells coming from a newly established arms producer that “...Read more

Senate candidate Steve Garvey: College campus protests over Gaza could trigger less federal aid to schools
Republican Senate candidate Steve Garvey strongly condemned campus protests critical of Israel’s war in Gaza and warned he’d push to take away federal aid to schools that appear to encourage dissent that’s antisemitic.
He told a Los Angeles news conference that “demonstrations that allow people to build encampments that obstruct the ...Read more

High-capacity gun magazines stay illegal in Washington state, court commissioner rules
SEATTLE — High-capacity magazines, those holding more than 10 bullets, will remain illegal to buy or sell in Washington, while the state appeals a lower-court ruling that found the ban unconstitutional, the state Supreme Court commissioner ruled Thursday.
Commissioner Michael Johnston, who acts as a gatekeeper before the court can fully ...Read more

At UC Irvine, hundreds turn out to demonstrate in support of Palestine
IRVINE, Calif. — During the two hours protesters at the University of California, Irvine chanted and marched on campus calling for the university to divest financial ties from Israel on Thursday, there was zero visible presence of officers in uniform.
That’s a far cry from the mass pro-Palestinian protest at the University of Southern ...Read more

Pecker at hush money trial says Trump feared trysts would hurt image, but didn't mention Melania
Donald Trump never mentioned worrying about his wife getting wind of his alleged trysts with porn star Stormy Daniels and Playboy model Karen McDougal — only his future in politics, a Manhattan jury heard Thursday during explosive testimony at the former president’s hush money trial.
During his third day on the witness stand, the former CEO...Read more

Illinois Gov. J.B. Pritzker pushes increased funding to fight racial disparities in homelessness
Gov. J.B. Pritzker used the release of a report on Black homelessness in Illinois to press the case for his proposal to increase funding on efforts to address the issue in next year’s budget by $50 million, which would go toward additional rental assistance, legal aid and new programs.
The report, a collaboration between the state’s Office ...Read more

US, allies press Hamas for 'immediate release' of hostages
The U.S. and 17 other nations pressed Hamas to release their citizens who are missing or held hostage in the Gaza Strip, in a bid to revive cease-fire talks that have stalled out in recent weeks and unlock more humanitarian aid.
The countries’ leaders released a joint statement Thursday intended to reflect mounting concern about the well-...Read more

As UN appeals for aid for Haiti, Pentagon sends in another military flight with supplies
Short on funding and with barely enough hot meals to last for the next six weeks, the World Food Program is appealing for humanitarian assistance for Haiti.
The organization’s deputy executive director, fresh off a visit to the Caribbean nation over the weekend, said with more than 1 million Haitians facing famine, the United Nations food aid...Read more

City College of New York becomes latest site of heated pro-Palestinian demonstrations
NEW YORK — Students set up a pro-Palestinian encampment Thursday at City College of New York, with one passerby being driven away when a protester claimed she could “smell” he was a “Zionist.”
Concern about antisemitism at protests sweeping campuses around the nation has grown in recent days, sparking demands for university officials ...Read more

Haiti enters new era of governing: Presidential council sworn in, prime minister resigns
Haiti entered a new era of governance on Thursday with the official installation of a new nine-member presidential council in a two-part ceremony in the country’s gang-ridden capital.
In a last-minute decision, members opted to secretly take the oath of office on the grounds of the National Palace — which has been under constant attack by ...Read more

Karen Read murder trial will be held in smaller courtroom
BOSTON — The Karen Read murder trial, which has become a hot-button case not only locally but for true crime lovers across the nation, will be held in a much smaller courtroom.
Norfolk Superior Court Judge Beverly Cannone also reiterated during a hearing Thursday that attorneys could not directly raise the federal probe into the Read ...Read more
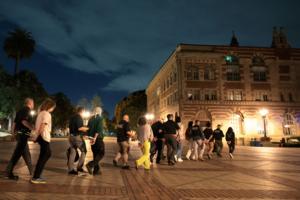
Pro-Palestinian protests grow at UCLA and UC Santa Barbara
LOS ANGELES — Pro-Palestinian protests grew Thursday at California colleges and universities, including a new encampment at UCLA and demonstrations at UC Santa Barbara, a day after police in riot gear arrested 93 protesters at USC.
Fallout over the Israel-Hamas war grew Thursday as USC announced that it would cancel its main stage ...Read more

Harvey Weinstein rape conviction overturned by NY appeals court; California conviction remains
In a dramatic reversal of the nation’s landmark #MeToo trial, a New York appeals court on Thursday overturned the sex assault conviction of disgraced movie mogul Harvey Weinstein.
The state appeals court found, in a 4-3 decision, that the judge who presided over Weinstein’s 2020 trial prejudiced his case by allowing four women who said ...Read more

Nevada boy with autism found in metal cage, parents arrested
LAS VEGAS — A Henderson, Nevada, couple were arrested after police found a boy with autism locked in a “makeshift jail cell” and their three other children living in a home smeared with feces, according to arrest reports.
Misty Scanlan, 46, and Jeffery Scanlan, 41, were booked into the Henderson Detention Center on Tuesday on one count of...Read more

Trump advisers discuss penalties for nations that move away from the dollar
WASHINGTON — Former President Donald Trump’s economic advisers are considering ways to actively stop nations from shifting away from using the dollar — an effort to counter budding moves among key emerging markets to reduce exposure to the U.S. currency, according to people familiar with the matter.
Discussions include penalties for ...Read more

'Bizarre': Why did Rand Paul miss the Ukraine foreign aid vote?
Kentucky’s U.S. Sen. Rand Paul was among three senators who missed Tuesday’s vote on the $95 billion foreign aid package providing weapons and equipment to Ukraine, Israel and Taiwan.
Two days later and his office won’t explain why.
The libertarian anti-interventionist was an outspoken critic of the supplemental spending measure ...Read more

Mass. Legislature sends Gov. Healey $426 million shelter bill that caps stays at nine months
BOSTON — Massachusetts lawmakers shipped Gov. Maura Healey a spending bill Thursday that hands her $426 million to spend on emergency shelters over the next year-plus and caps families’ time in the system at nine months.
The dollars come at a crucial moment for the Healey administration, which has warned for months that cash to pay for ...Read more

Pecker at hush money trial says Trump feared trysts would hurt image, but didn't mention Melania
NEW YORK — Donald Trump never mentioned worrying about his wife, Melania, getting wind of his alleged trysts with porn star Stormy Daniels and Playboy model Karen McDougal — only his future in politics, a Manhattan jury heard Thursday during explosive testimony at the former president’s hush money trial.
During his third day on the ...Read more

Columbia facing lose-lose decision as Gaza protest encampment deadline looms; Ilhan Omar on campus
NEW YORK — As a deadline for Columbia University administrators and protesters to iron out a deal to clear a Gaza encampment edges closer, university officials are facing a lose-lose decision if students continue to flout school rules: Call in the NYPD for a second time or allow a situation they have said cannot continue to continue.
...Read more
Popular Stories
- Israel-Hamas war protesters clash with officers at Emory in Atlanta
- Supreme Court sounds conflicted over Trump criminal immunity
- 'Bizarre': Why did Rand Paul miss the Ukraine foreign aid vote?
- USC cancels 'main stage' commencement
- Harvey Weinstein rape conviction overturned by NY appeals court; California conviction remains