Current News
/ArcaMax

Tufts encampment supporters say they'll boycott commencement if officials turn to police
BOSTON — If Tufts University calls on the police to break up a pro-Palestinian encampment on campus, students behind the movement say they’ll boycott commencement.
Roughly 320 undergraduate and graduate students set to receive their degrees later this month have sent a letter to university President Sunil Kumar urging Tufts to stay away ...Read more

Arizona Senate votes to repeal 1864 abortion law, leaving state with 15-week ban
The Arizona Senate voted Wednesday to repeal the state's 1864 abortion ban, sending a measure to the Democratic governor that would end weeks of turmoil and keep in place a 15-week abortion limit enacted in 2022.
In a testy and emotional session that included angry spats, a senator reading from the Bible and another playing a recording of his ...Read more
Missouri Capitol grinds to a halt as hard-right senators block billions for Medicaid
KANSAS CITY, Mo. — A protracted filibuster from a group of hard-right senators has ground the Missouri Senate to a halt, threatening to derail the General Assembly’s final weeks of session.
The state’s Medicaid program and a roughly $50 billion budget that funds state operations hang in the balance. The results could be devastating to ...Read more

Ex-adviser to Michigan Gov. Rick Snyder files intent to sue AG over Flint water case, claims malicious prosecution
LANSING, Mich. — A former adviser to Republican Gov. Rick Snyder intends to sue the attorney general, former solicitor general and the Wayne County prosecutor on allegations that the unsuccessful criminal case pursued against him stemming from the Flint water crisis amounted to malicious prosecution.
Richard Baird, who served as Snyder's ...Read more

Thiru Vignarajah drops out of Baltimore mayoral race, endorses Sheila Dixon
BALTIMORE — With less than 24 hours remaining before early voting begins, Baltimore mayoral candidate Thiru Vignarajah withdrew his candidacy Wednesday and endorsed rival Sheila Dixon, potentially shifting votes on the eve of the election.
His late exit from the Democratic race is the first time Vignarajah, a repeat candidate for both mayor ...Read more

DA will retry Harvey Weinstein after appeals court overturns NY rape conviction
NEW YORK — Manhattan prosecutors on Wednesday told a judge they will retry disgraced film producer Harvey Weinstein following the overturning of his rape and sexual assault conviction and will be ready for trial as soon as this fall.
The decision was announced by prosecutors from Manhattan District Attorney Alvin Bragg’s office at a hearing...Read more

Over 100 pro-Palestinian protesters arrested during NYPD raid of Columbia University
NEW YORK — Over 100 pro-Palestinian protesters were arrested when an army of NYPD cops stormed Columbia University to end the seizure of a school building where all the doors had been barricaded with bicycle locks, officials said Wednesday.
Officers, some wielding chainsaws, climbed in through windows Tuesday night to enter Columbia’s ...Read more

NYPD cops hoist US flag at City College after school worker tosses away Palestinian flag
NEW YORK — Cops including two high-ranking NYPD officials proudly hoisted an American flag — and a school worker pulled down and tossed away a Palestinian flag — after evicting protesters encamped at City College, video posted by the department shows.
Kaz Daughtry, the NYPD’s Deputy Commissioner for Operations, posted the video early ...Read more

Mitt Romney insists he's no dog killer, unlike South Dakota Gov. Kristi Noem
Mitt Romney says he resents being compared to South Dakota Gov. Kristi Noem after she bragged about killing a pet dog over its troublesome behavior.
The former Republican presidential standard-bearer insisted that he did nothing nearly so cruel as Noem by strapping a pet dog to the roof of his car, a much-mocked episode from his 2012 White ...Read more

Biden rips Trump over abortion as draconian Florida ban goes into effect
President Biden ripped former President Trump over abortion on Wednesday, the same day a draconian ban went into effect in Florida.
Biden derided his White House rival for giving his blessing to Republican-run states that have enacted ever-stricter restrictions on abortion since the conservative Supreme Court rolled back the Roe v. Wade ...Read more

UCLA cancels classes after counterprotesters violently attack pro-Palestinian camp
LOS ANGELES — University administrators canceled classes at UCLA on Wednesday, hours after violence broke out at a pro-Palestinian encampment set up on campus.
Just before midnight, a large group of counterdemonstrators, wearing black outfits and white masks, arrived on campus and tried to tear down the barricades surrounding the encampment. ...Read more

Florida's new abortion ban goes into effect, but voters will decide its future
MIAMI — Democrats and abortion-access advocates have been warning for months of an impending voter backlash against Republicans who backed Florida’s six-week ban on the procedure. Now, it’s time to find out whether they’re right or wrong.
The six-week ban, which was quietly signed into law last year by Gov. Ron DeSantis, went into force...Read more

Marjorie Taylor Greene to call for vote on Speaker Johnson's ouster next week
WASHINGTON — U.S. Rep. Marjorie Taylor Greene said she is ready to call for a vote to remove Mike Johnson as House speaker, even if it ultimately fails with Democrats’ help.
Greene, at times angry and cursing during a Wednesday morning news conference on the steps of the U.S. Capitol, said Johnson is a weak leader who has allowed the ...Read more
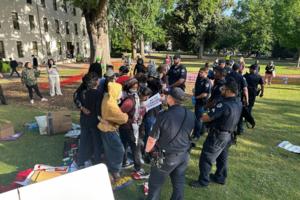
University of Georgia police report includes more protest arrest details
ATLANTA — Nine University of Georgia students were among the 16 people arrested Monday for trespassing at an Israel-Gaza protest on the university campus, according to the UGA police department.
The Atlanta Journal-Constitution previously reported 10 students were arrested. However, one person was a UGA graduate, not a current student, ...Read more

United Methodists strike decades long ban on ordination of LGBTQ clergy
There were tears, hugs, prayer and singing in Charlotte, North Carolina, after the United Methodist Church removed a line prohibiting the ordination of LGBTQ clergy that had been in place since 1984.
According to Unite Methodist News, delegates - without debate - voted 692 to 51 for the changes on the day’s consent calendar, which enabled the...Read more

Probe of NYC mayoral adviser Timothy Pearson broadens in wake of harassment allegation
NEW YORK — A city Department of Investigation probe of mayoral adviser Timothy Pearson has been expanded to include his role in NYPD personnel and promotional matters concerning officers involved in two lawsuits accusing him of sexual harassment and retaliation, the New York Daily News has learned.
DOI probers have asked the NYPD for a wide ...Read more

Feds announce student loan relief for former Art Institute students
The White House announced Wednesday the approval of more than $6.1 billion in automatic student loan relief to nearly 317,000 borrowers who enrolled at any Art Institute campus between January 2004 through October 2017.
The U.S. Department of Education found that The Art Institutes and its parent company, Education Management Corporation, “...Read more
Fed flags lack of inflation progress but signals hikes unlikely
The Federal Reserve signaled fresh concerns about inflation while indicating it was likely to keep borrowing costs elevated for longer rather than raising them again.
Officials unanimously decided Wednesday to leave the target range for the benchmark federal funds rate at 5.25% to 5.5% — where it’s been since July — following a slew of ...Read more

Amid continued demonstrations, swastika drawn on USC's campus
LOS ANGELES — In the midst of tense demonstrations at USC over the war in Gaza, officials found a swastika drawn on a campus fence.
"Clearly it was drawn there just to incite even more anger at a time that is so painful for our community," USC President Carol Folt wrote in a statement on Instagram. "I condemn any antisemitic symbols or any ...Read more

Mexico emerges as a destination for Americans seeking reproductive health services – not for the first time
When its six-week abortion ban went into effect on May 1, 2024, Florida joined nearly two dozen other U.S. states that ban abortion or greatly restrict it.
These laws came into effect after the Supreme Court’s 2022 decision to overturn Roe v. Wade ended nearly 50 years of the constitutional right to abortion in the United States....Read more
Popular Stories
- Climbers have turned Mount Everest into a high-altitude garbage dump, but sustainable solutions are within reach
- The biblical character who goes ‘down the rabbit hole’ into an alternate reality − just like Alice in Wonderland
- Electric air taxis are on the way – quiet eVTOLs may be flying passengers as early as 2025
- For the ancient Maya, cracked mirrors were a path to the world beyond
- To reduce Black-on-Black crime, two criminal justice experts explain why offering monthly stipends to people at risk makes sense





