Current News
/ArcaMax

'We will not be leaving': UNC students camp out to protest Israel-Hamas war
CHAPEL HILL, N.C. — Students and others pitched tents on the campus of the University of North Carolina, Chapel Hill Friday, calling on the university “to divest from the ongoing genocide in Gaza” and forming an encampment similar to others on college campuses nationwide.
The event, which began around 10 a.m., was organized by the UNC ...Read more

Transgender students would be banned from preferred bathroom under South Carolina Senate proviso
COLUMBIA, S.C. — Biological girls and boys can not enter the opposite sex’s bathroom in public schools under a new South Carolina state Senate proviso that passed Wednesday. This means transgender students in South Carolina could be prohibited from using bath, locker or changing rooms that align with their gender identity.
Democratic state ...Read more

Columbia University's ongoing talks over pro-Palestinian encampment slammed by Jewish campus leader
NEW YORK — Columbia University officials and the student protesters behind a pro-Gaza encampment negotiated Friday past a school-set deadline to clear the schools’s main lawn — much to the dismay of a leading Jewish campus organization.
Brian Cohen, the Lavine Family executive director of Hillel, was sharply critical of the university ...Read more

Mexican presidential candidate Xochitl Galvez readies for a must-win debate
MEXICO CITY — Mexico’s opposition presidential candidate Xochitl Galvez is running out of opportunities to mount a serious challenge against President Andres Manuel Lopez Obrador’s anointed successor, trailing by a wide margin in polling just five weeks before election day.
On Sunday, in the second of three debates against front-runner ...Read more

Biden enacts most sweeping reform of gun exports in decades
WASHINGTON — To curb the use of U.S.-made civilian guns in crimes and human rights abuses abroad, the Biden administration will require exporters to better vet their customers and tighten sales to 36 countries deemed “high-risk” for illicit diversion of semiautomatic firearms.
The new rules — the most sweeping in decades — also cut ...Read more

University of Central Florida students rally for Palestinians, the latest in nationwide campus divestment protests
ORLANDO, Fla. — As protests roil college campuses nationwide, University of Central Florida students and local activists rallied Friday afternoon in solidarity with Palestinians and against the war in Gaza.
More than 200 gathered in front of the John C. Hitt Library, many carrying Palestinian flags and wearing keffiyehs as they called for an ...Read more

OJ Simpson's cause of death confirmed
O.J. Simpson’s cause of death has officially been confirmed, just weeks after the former football star’s death at the age of 76.
Simpson — who had long been just as famous (if not more so) for his double-murder trial as his acclaimed athletic career — died of metastatic prostate cancer on April 10, his attorney and executor of his ...Read more

Kansas House votes to overturn Gov. Laura Kelly's tax cuts veto, with many Democrats joining Republicans
The Kansas House on Friday voted to override Democratic Gov. Laura Kelly’s veto of a sweeping tax package, which would eliminate one of the state’s income tax brackets, that she called too expensive.
Numerous Democrats joined Republicans to overturn the veto in a 104-15 vote. The override now heads to the Senate, where the legislation ...Read more

Tensions with insurers mount in Archdiocese of Baltimore bankruptcy case
BALTIMORE — Tensions are growing in the Archdiocese of Baltimore’s bankruptcy case around the role of the church’s insurers.
Baltimore’s arm of the Catholic Church, America’s oldest archdiocese, has sued its insurers for alleged breach of contract in one legal battle. In another argument, the insurers sought to block the committee of ...Read more

Trump at hush money trial wishes Melania 'happy birthday' as Pecker testimony about trysts continues
NEW YORK — Donald Trump wished his wife Melania a happy birthday walking into his hush money trial Friday — a day after former National Enquirer publisher David Pecker said the former president never mentioned worrying about what she thought about his alleged affairs at the heart of the state’s case against him.
“I want to start by ...Read more

King Charles to resume public duties next week, despite reports of funeral planning, says palace
King Charles has progressed enough in his cancer treatment that he’ll return to public duties next week for the first time since announcing his diagnosis, Buckingham Palace said Friday.
The news comes just one day after unnamed sources told The Daily Beast that the 75-year-old British monarch was “significantly more unwell than his aides ...Read more

White House drops plan to ban menthol cigarettes
WASHINGTON — The Biden administration on Friday announced it is dropping — for now ― a plan to ban menthol cigarettes after months of speculation about the proposal’s future.
Health and Human Services Secretary Xavier Becerra did not say when or if the administration would revisit the issue, nor did he mention the fate of a related ...Read more

Trump 'hush money' trial: Key takeaways from week one
The opening week of Donald Trump’s historic hush money trial offered plenty of insights into how his 2016 presidential campaign teamed up with a tabloid publisher to silence embarrassing stories from women who said they’d had extramarital affairs with him.
David Pecker, the former chief executive of the firm that published the National ...Read more

Athletic director at Maryland high school used AI to fake racist recording of principal, police say
BALTIMORE — Pikesville High School’s athletic director was arrested Thursday morning in connection with an artificial intelligence-made audio clip of the school’s principal having a fake, racist conversation.
Dazhon Darien, 31, is charged with disrupting school activities after Baltimore County Police say he created the falsified audio ...Read more

US Defense Chief confirms $6 billion arms commitment for Ukraine
WASHINGTON — Defense Secretary Lloyd Austin confirmed Friday a $6 billion commitment for long-term contracts to provide Ukraine with weapons such as Patriot missiles, artillery ammunition and drones.
Austin laid out a broad commitment but not signed contracts, which would be likely to take months to complete under the Ukraine Security ...Read more

Ex-American Airlines flight attendant accused of filming girl in Boston-bound plane's bathroom indicted
BOSTON — A former American Airlines flight attendant who’s accused of filming a girl in a plane’s bathroom on a Boston-bound flight has been indicted, according to the feds.
Estes Carter Thompson III, 36, of Charlotte, North Carolina, also allegedly had recordings of four other girl passengers using airplane lavatories.
Thompson was ...Read more
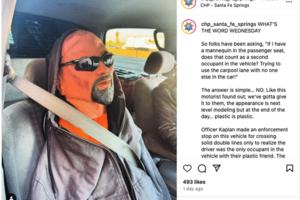
Carpool lane driver in LA caught with mannequin in the passenger seat
LOS ANGELES — Los Angeles traffic is so notoriously bad, some residents might be willing to do anything to cut down on wait time — including driving with a mannequin in the passenger seat.
A California Highway Patrol officer in Santa Fe Springs stopped a driver recently for crossing solid double lines to enter a carpool lane, only to bust ...Read more

Kim Kardashian meets with VP Harris, pardoned prisoners at White House
Kim Kardashian made a return to the White House this week to discuss criminal justice reform with Vice President Kamala Harris.
The reality TV star — and billionaire business mogul — joined a roundtable discussion Thursday regarding the 11 pardons issued and five sentences commuted by President Joe Biden the day prior.
Kardashian was ...Read more

KC police officer who ran anti-crime charity indicted for wire fraud, money laundering
KANSAS CITY, Mo. — A Kansas City police officer has been indicted on 14 federal counts of wire fraud and two counts of money laundering, the U.S. Attorney’s Office for the Western District of Missouri announced Friday.
Aaron Wayne McKie, 46, has been a Kansas City Police Department officer since April 2000.
The charges allege he took ...Read more

Buckingham Palace, UK government reviewing 'unwell' King Charles' funeral plans, report says
Buckingham Palace and the British government began reviewing hundreds of documents related to King Charles III’s future funeral following the monarch’s cancer diagnosis, according to a report.
Little is known about Charles’ mysterious illness outside of his initial announcement of “a form of cancer” in February, but sources close to ...Read more
Popular Stories
- At UC Irvine, hundreds turn out to demonstrate in support of Palestine
- High-capacity gun magazines stay illegal in Washington state, court commissioner rules
- Pecker at hush money trial says Trump feared trysts would hurt image, but didn't mention Melania
- Emerson College protest arrests divides along public safety vs political lines
- City College of New York becomes latest site of heated pro-Palestinian demonstrations





