Abortion drug access could be limited by Supreme Court − if the court decides anti-abortion doctors can, in fact, challenge the FDA
Published in Political News
Who has the legal right to challenge decisions by the U.S. Food and Drug Administration? And should the moral umbrage of a group of anti-abortion rights doctors shift policy across the country, limiting women’s ability to get the widely used abortion drug mifepristone?
These are a few of the central questions that the Supreme Court fielded on March 26, 2024, during the oral arguments in FDA v. Alliance for Hippocratic Medicine. A group of doctors is challenging the FDA, saying that the federal agency’s decisions allowing people to get mifepristone via telehealth, at up to 10 weeks of pregnancy, is causing some medical professionals harm.
Amy Lieberman, politics and society editor at The Conversation U.S., spoke with family law and reproductive justice scholars Naomi Cahn and Sonia Suter to better understand what’s behind the oral arguments before the Supreme Court – and how the court’s eventual decision, expected in June, could affect people’s ability to get abortions by using mifepristone, one of two drugs used for medication abortion.
What is this case about?
Sonia Suter: It’s about whether the FDA’s regulations for the use of mifepristone were appropriately loosened in 2016 and 2021. These changes generally make mifepristone more accessible by allowing people to have the medication prescribed via a telehealth visit and then getting the pill in the mail.
Naomi Cahn: That 2016 regulation also extended the time during which mifepristone could be prescribed, increasing it from seven to 10 weeks gestation. Medication abortions accounted for 63% of all abortions that occurred in the U.S. in 2023. This percentage has increased since the Supreme Court overturned the constitutional right to an abortion in 2022.
Why are these guidelines being challenged?
Suter: A group of doctors and medical associations that oppose abortion are challenging these guidelines and using this court case as a way, we believe, to limit the ability to get an abortion by using medication.
They challenged the drug’s initial approval by the FDA and the relaxed restrictions on how it is used. They claimed that the FDA exceeded its authority, did not rely on proper data and did not have adequate support from scientific studies for its decision that mifepristone could be safely prescribed. Their initial arguments, which the lower court accepted, would have banned mifepristone. But that decision was not upheld by the 5th Circuit Court.
Instead, the issues before the Supreme Court focus on whether the FDA should have expanded the use of mifepristone. Virtually all studies have shown that mifepristone is not dangerous, even with the relaxed conditions on its use.
...continued

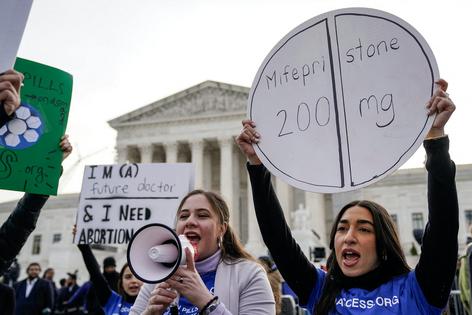











Comments