Recipes
/Home & Leisure

EatingWell: Skillet dinner delivers on flavor, easy cleanup
In this healthy ground beef and potatoes recipe, ground beef and potatoes are paired with colorful veggies, including kale, tomato and peppers. Everything is cooked in one skillet, which allows for layers of flavor to build quickly while also cutting back on the number of dishes.
Ground Beef & Potatoes Skillet
Serves 6
Active Time: 40 minutes...Read more

Environmental Nutrition: Yogurt and yogurt drinks
Yogurt is so much more than it once was. These days there’s fat-free, low-fat, and whole milk varieties as well as yogurt alternatives made from soy milk, cashew milk, almond milk and coconut milk. Choose drinkable yogurts when you are on the go.
Most choices are loaded with nutritional benefits — think protein and calcium — and there are...Read more

Seriously Simple: This roast chicken dish is a springtime favorite
I am always looking for a Seriously Simple chicken recipe that can make it into my cooking rotation. It must be both kid-friendly and good enough to serve at a casual dinner party. This recipe satisfies those conditions.
I think of this as a springtime recipe, with the addition of artichokes that make their arrival in early spring. As a ...Read more

JeanMarie Brownson: Brunch is the perfect place for carbs
Freshly baked bread is our family’s love language. We nearly always have a beautiful loaf on the menu, no matter the season. During the cold weather, patience comes easily for the long, slow rises of homemade sourdough loaves. As the days lengthen, we turn to quicker rises, brighter flavors, and lighter texture.
Both of the breads that follow...Read more

The Kitchn: My mom’s clever trick will forever change the way you eat banana bread
My mom has made banana bread so often that she can make it without even referencing a recipe. And I’m sure she’s not alone. The sweet but simple loaf is easily customizable and requires little prep — it’s no wonder it’s ...Read more

The Kitchn: The ‘French 75’ cocktail will make you feel like you’re at a fancy restaurant
When I want a light cocktail before dinner or a drink to go with brunch, I always opt for the elegant French 75 — not a classic mimosa. With a bright, sunny color, this sparkling, lemon-y cocktail is refreshing, crisp, and bright, with subtle notes of herbs from the...Read more

A Salvadoran cookbook from a major publisher is finally here. Why did it take so long?
LOS ANGELES -- Food never happened in a vacuum for Karla Tatiana Vasquez. Stories always followed.
Whenever her grandmother or mother cooked, Vasquez knew something special was coming. Their food unlocked memories, especially about El Salvador — the homeland they'd fled in the late 1980s during the country's civil war.
Vasquez was born in ...Read more

Want to cook vegetables better? The new Kismet cookbook shows us how
LOS ANGELES — Call them the vegetable whisperers. Sarah Hymanson and Sara Kramer are the two produce-obsessed chefs behind Kismet restaurant in Los Feliz, the growing chainlet of Kismet Rotisserie takeout shops (there are three across L.A. now, where the tahini roasted cauliflower is as popular as the chicken) and a new vegetable-forward ...Read more

The recipe that will get you hooked on tofu
Tofu is a wonderfully versatile ingredient. It's great pan-fried, grilled, scrambled and whizzed into spreads and sauces. But I like it best pan roasted until it becomes meltingly golden and crisp. For this, you need a really good tofu, and the fresher the better.
Minnesota-made tofu is tastier than the tofu from California. MinnTofu, found in ...Read more

Joan Nathan is more than a Jewish cookbook writer. Her new memoir reveals why
LOS ANGELES -- The morning after Joan Nathan's 80th birthday party last year — a long-table Palm Springs gathering on the grounds of a hacienda-style compound where Samuel Goldwyn, Lucille Ball and Judy Garland are said to have lived at various times — guests from the nighttime celebration were invited to brunch at the midcentury showcase ...Read more

The secret to French onion ramen and other life lessons from 'The Mythical Cookbook'
LOS ANGELES -- Earlier this month Josh Scherer cooked a corned beef sandwich and a few gelatin-encased shrimp in a nod to foods that have been to space. Earlier this year he served Gordon Ramsay a beef Wellington, a deep-fried Mars bar, an In-N-Out burger and everything else the chef decreed would be his ideal last meal on Earth. He's re-created...Read more

A new Mediterranean cookbook from José Andrés celebrates 'dishes that belong to the people'
LOS ANGELES -- José Andrés spends much of his time contemplating the unifying nature of food, both in and out of the world's most dangerous conflict and disaster zones.
Days before an Israeli airstrike killed seven members of his aid organization working to feed Palestinians in Gaza, Andrés spoke to The Times about his recently published ...Read more

This is the best Italian dish you've never heard of
Chicken Scarpariello is a beloved classic Italian-American dish, and I'd never heard of it.
I came across this delicious sounding sweet-and-sour chicken, with sausages and fresh and pickled peppers, in the process of researching another recipe. It sounded so good that, like a dog chasing a squirrel, I forgot all about my original project and ...Read more

How to make Michael Twitty's matzoh ball gumbo, a soup of Black and Jewish cuisines
LOS ANGELES -- With a rainbow knitted kippah affixed to the crown of his head, Michael Twitty is paced and intentional as he moves through the test kitchen at the L.A. Times. He's here to make one of his classic Passover dishes, matzoh ball gumbo, which combines two recipes from his food memoir "Koshersoul."
The crimson stew adds buoyant, ...Read more

Gretchen's table: Chicken shawarma in a bowl is a tasty, healthy meal
Protein bowls are appealing for many reasons, the biggest of which is they're incredibly versatile.
Whether you top them with a lean meat like roasted chicken or a fatty, good-for-you fish like salmon — or opt for a vegetarian source of protein such as tofu or canned chickpeas — bowls can fill you up with countless combinations, while also ...Read more

Daniel Neman: We're surrendering our privacy for a bag of M&Ms
I am now at the age when I am convinced that the world is going to Hades in a handbasket.
And I have proof.
Obviously, this is not a new or unique idea. People of a certain age — my certain age — have been making the Hades-handbasket connection from time immemorial. Even Pollyanna, in her later years, was saying, “They just don’t make ...Read more

Best Bites: Sweet Street Salted Caramel Manifesto Cookie
My son recently brought this cookie home from college. “You have to try it,” he told me, “It’s so good.”
He was certainly right.
The salt and the caramel are not afterthoughts but rather almost what you taste before the buttery cookie part. Somehow, the edges taste like you just scraped a little caramel off a cookie sheet. One ...Read more
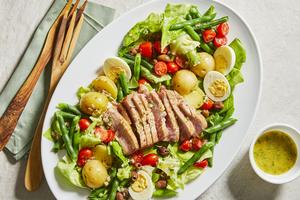
EatingWell: Hearty salad perfect for spring menu
This salad features all the classic niçoise ingredients and flavors, with a bright and fresh lemony dressing, and uses fresh seared tuna in place of canned. The tuna will be rare in the center. Add an additional 2 to 3 minutes per side for more doneness.
Tuna Niçoise Salad
Serves 4
Active Time: 45 minutes
Total Time: 50 minutes
10 ounces ...Read more

Environmental Nutrition: There’s something about sauerkraut
“Sauerkraut” is simply the German word for sour cabbage.
The folklore
Although sauerkraut is considered to be a national dish of Germany, and has been a staple of the German diet since the 1600s, it didn’t originate in Germany. It is believed to have originated in China and was then brought to Europe. Originally, ...Read more

Seriously Simple: Spice up your meatballs with Korean gochujang sauce
Sometimes I need to shake things up in the kitchen. Meatballs with tomato sauce and plenty of freshly grated Parmesan cheese is a favorite of mine. Recently, I decided to improvise on that recipe and add spicy sweet gochujang to the basic meatball mixture along with shredded carrots for juiciness and sesame oil and ginger — a wonderful medley ...Read more