Gardening
/Home & Leisure
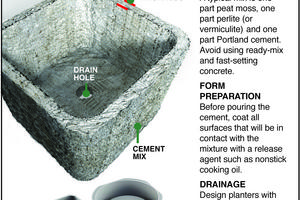
The Greener View: Hypertufa Planter
Q: Recently, I was reading an old garden magazine at the doctor's office about making your own concrete planter. I was going to ask about copying it, but I forgot and now the magazine is not there. Do you know what the ingredients are and how to make a concrete planter?
A: I do know, and it is a project that many gardeners can complete. First, ...Read more

The Greener View: Growing Bell Peppers
Q: I love red and purple bell peppers, but I can't seem to grow them in my garden. I buy them at a local garden center. They look healthy, but at the end of the summer, I only get a few peppers from each plant.
A: Bell peppers are one of the most-planted vegetables, and I have heard your complaint before. Many garden vegetables start producing ...Read more

The Greener View: Gypsum
Question: I recently moved to a new home, and I thought the lawn looked like it needed some help, so I went to the store for some gypsum. Where I used to live, gypsum was added to the lawns all the time. I was told that in my new region, no one adds gypsum, but some people add limestone. What are gypsum and limestone used for? What can I do to ...Read more

The Greener View: Easter Lilies
Easter is Sunday, March 31, this year. Last year it was April 9, and next year it won't be until April 20. Even though Easter lilies have only a two-week moving sales window related to the date that Easter falls, they are the fourth-largest potted plant crop behind poinsettias, mums and azaleas. Even though we see them for such a short time, ...Read more

The Greener View: Crabgrass and Dormant Oil
Question: Does dead crabgrass look like white dead clumps dotted around the lawn? If so, should one dig each one up and fill in with fescue seed, fertilizer and dirt?
Answer: At this time of year, all dead annual and many dormant perennial grasses look like white dead clumps. If the good grass in your lawn has some signs of green and there are ...Read more
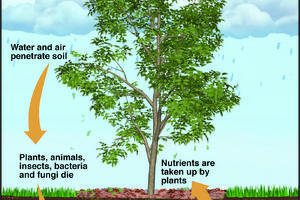
The Greener View: Climate Change and Trees
Q: We have a new landscape, and we want to plant trees that will help with climate change. Any suggestions?
A: What is your definition of climate change? Do you think your area is going to get warmer or colder? What are you trying to accomplish by planting a tree? Are you planting the tree to absorb carbon or to shade the house? Will a tree ...Read more

The Greener View: Purple Tomatoes
Home gardeners can finally grow their own genetically engineered vegetable crop. Before I tell you what it is, we need to define the terms. "GMO" is a widely misused term. It stands for genetically modified organism. In the United States, this term has no legal definition. Many people think they are against anything that is genetically modified,...Read more

The Greener View: Florel the Little Chemical that Could
Q: Didn't you write about a product that could stop all the stinky ginkgo fruit from falling off my trees? I think it was supposed to be applied in the spring, and spring is coming soon. I don't want to cut down my two ginkgo trees, but the fruit makes a huge mess in the fall.
A: I think you are right. The product is called Florel, and as far ...Read more
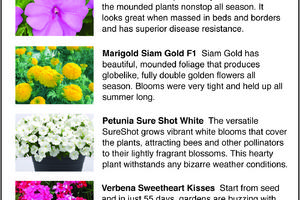
The Greener View: More All-America Selections Flower Winners
Finding the red, white and blue logo of All-America Selections on seed packets, bedding plant tags or catalogs will help you find flowers and vegetables that will grow great in your garden. Last week, we discussed the three vegetable winners for 2024 and three of the flower winners. Today, we finish with five more flower winners.
The judges ...Read more

The Greener View: 2024 All-America Selections Winners
One way that I know a plant could work well in my garden is that I look for the red, white and blue logo of All-America Selections on seed packets, on bedding plant tags or in catalogs. Even AAS winners from several years ago are more likely to prove successful than nonwinners.
The AAS testing program is an independent nonprofit organization ...Read more

The Greener View: Valentine Flowers
I have a few questions about Valentine's Day, and maybe you do too. Why do they sell "I only love you" cards in a 10-pack? What is Galentine's Day? I can't answer the first question, but Galentine's Day is the day before Valentine's Day, and it is the day ladies celebrate their girlfriends.
No matter if you celebrate one or both days, flowers ...Read more
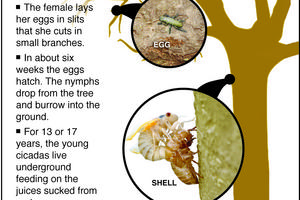
The Greener View: Are Cicadas Going To Be a Problem This Year?
Q: We were planning on planting several new trees this spring, but we were told by some friends that this summer, cicadas are coming, and they could kill small trees. Do you think is it worth the effort to plant trees this year, or should we wait?
A: I say plant away. Trees are not made in a factory when you want one. The tree you want to plant...Read more

The Greener View: American Garden Rose Selection 2024 Winners
The American Garden Rose Selections (AGRS) judges have announced the newest roses to be selected in their testing program. This year there are three new winners.
The All America Rose Selection program ran from 1940 until 2013. The AGRS program began in 2016. Roses that have been selected in either program are the best ones for gardeners to try....Read more






