Business
/ArcaMax

Boeing workers still scared to raise safety concerns, say FAA-appointed experts
After spending a year interviewing Boeing employees and executives, documenting the company’s safety processes and taking stock of changes Boeing made after two deadly 737 Max crashes, an expert panel determined the company has much more work to do.
Among its many findings, the panel concluded Boeing employees fear retaliation if they speak ...Read more

Boeing hid safety risks in 'criminal cover-up,' whistleblowers tell Senate
In sworn testimony before a U.S. Senate hearing Wednesday, a Boeing engineer reiterated his accusation that Boeing has hidden safety risks on the 787 Dreamliner and the 777 widebody jets, rejecting the account Boeing provided Monday in an effort to reassure the public.
And a former Boeing manager accused the company of a “criminal cover-up”...Read more
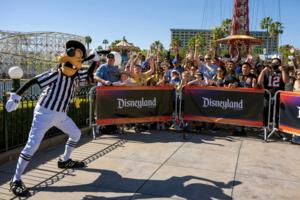
Disneyland costumed staffers from Goofy to Elsa seek union vote
Walt Disney Co. employees who dress up as Goofy and other beloved characters to entertain guests at the company’s California theme parks have taken a key step toward unionizing.
A majority of the staff members who meet with fans or participate in parades at the Disneyland Resort have signed a petition indicating they want to join the Actors�...Read more

As other banks report decline in deposits, US Bancorp experiencing a surge
As other banks report declines to their average deposits, Minneapolis-based U.S. Bancorp, the parent company of U.S. Bank National Association, has experienced a surge in that segment despite an uncertain rate environment.
For the bank's first quarter ended March 31, average total deposits increased $279 million, or 0.1%, from the fourth ...Read more

Tech layoffs jolt Bay Area economy with hundreds of new job cuts
SUNNYVALE, California — A high-profile aerospace and defense contractor and a semiconductor company were among the latest tech firms to chop jobs in the Bay Area, cutbacks that will erase more than 200 positions.
Lockheed Martin, Coherent, and Hinge Health, a tech company that provides online physical therapy, have decided to slash employment...Read more

Canada to start taxing tech giants in 2024 despite US complaints
Canada will start applying a proposed tax on the world’s biggest technology companies this year, despite threats from American lawmakers to carry out trade reprisals against a levy that will primarily hit U.S. firms.
Legislation to enact the digital services tax is currently before Canada’s Parliament. Once it passes, “the tax would begin...Read more

Boeing workers still scared to raise safety concerns, say FAA-appointed experts
After spending a year interviewing Boeing employees and executives, documenting the company’s safety processes and taking stock of changes Boeing made after two deadly 737 MAX crashes, an expert panel commissioned determined the company had more work to do.
Among its many findings, the panel concluded Boeing employees fear retaliation if they...Read more

Tesla asks investors to approve Musk's $56 billion pay again
Tesla Inc. will ask shareholders to vote again on the same $56 billion compensation package for Chief Executive Officer Elon Musk that was voided by a Delaware court early this year.
In its proxy filing issued Wednesday, Tesla also said it will call a vote on moving the company’s state of incorporation to Texas from Delaware. The carmaker ...Read more

Buy Nothing meets GoFundMe: How a new website aims to 'revolutionize' philanthropy
An Minnesota nonprofit leader hopes to "revolutionize" charitable giving with a new platform for people to trade household items they no longer need, resulting in a donation to a nonprofit.
Think Buy Nothing meets GoFundMe.
Joel Ackerman, a former UnitedHealth Group information technologies executive who's worked at numerous startups, was ...Read more

Tech review: Dyson is a champ at purifiying the air and keeping you comfortable
I sleep with a fan blowing to move the air in my bedroom.
Over the years I’ve had all sorts of fans, but for the last few years, my choice has been a Dyson fan sitting on a small table.
The Dyson fan I’ve been using was the Pure Hot+Cool. The Pure meant it had a HEPA filter, and Hot+Cool means it’s a heater as well as a fan. I loved that...Read more

Broken and unreliable EV chargers become a business opportunity for LA's ChargerHelp
Right place, right time, with an eye for opportunity, a commitment to economic growth for all, and a will to get things done. That’s entrepreneur Kameale Terry, co-founder of ChargerHelp, a Los Angeles startup.
She’s tackling a modern problem — the sorry state of electric vehicle public charging stations— while training an often-...Read more

Ford F-150 Lightnings now shipping after February stop order
Ford Motor Co. says it's shipping 2024 F-150 Lightning electric pickup trucks, and the vehicles now are available to order online.
The automaker on Feb. 9 had placed a stop-shipment on the all-electric trucks made at the Rouge Electric Vehicle Center in Dearborn because of an undisclosed issue resulting in the company saying it was extending ...Read more

Walgreens embarks on another round of layoffs
Walgreens confirmed Tuesday a new round of layoffs, the latest in a string of job cuts over the past year as the retail pharmacy giant aims to slash costs.
Walgreens spokesman Fraser Engerman said the company is not disclosing the number of people it’s laying off. Most of the positions are Chicago-based, he said in a statement.
“To ensure ...Read more

Dozens of Google employees protest use of company's tech for war in Gaza
SUNNYVALE, California — A group of disappointed and angry Google employees protested outside a company building on Tuesday after it was reported that the search giant had deepened a contract with the Israeli government.
Googlers in New York City and Sunnyvale took action with the activist group No Tech for Apartheid, demanding that the ...Read more

'Threaten our jobs and values': Southern politicians ramp up campaign against UAW organizing
Political opposition to the United Auto Workers’ southern organizing push is cranking up ahead of a first test of the union’s strength this week at Volkswagen AG’s Tennessee plant, where a worker vote on whether to join the union runs Wednesday to Friday.
A Tuesday joint statement from the Republican governors of Alabama, Tennessee, ...Read more

False offers of cash subsidies used to 'capture' health insurance customers, lawsuit alleges
A health insurance operation based in Broward County, Florida, used internet ads that falsely promised cash subsidies to sign up clients across the country and replace their agents, a lawsuit contends.
The scheme was carried out by Enhance Health LLC, TrueCoverage LLC, Speridian Technologies LLC, Number One Prospecting LLC and two individuals ...Read more

Cruise demand leaves pandemic in rearview with record passengers, more construction on tap
MIAMI BEACH — The COVID-19 pandemic drove the cruise industry to a standstill, but numbers released Tuesday signal the years of comeback are officially over with more expansion on tap.
More than 31.7 million passengers took cruises worldwide in 2023, said Kelly Craighead, Cruise Line International Association president and CEO, speaking at ...Read more

Powell signals rate-cut delay after run of inflation surprises
Federal Reserve Chair Jerome Powell signaled policymakers will wait longer than previously anticipated to cut interest rates following a series of surprisingly high inflation readings.
Powell pointed to the lack of additional progress made on inflation after the rapid decline seen at the end of last year, noting it will likely take more time ...Read more

Some Target Up & Up acne products subject of federal class action suit
Target is facing a class action lawsuit after a Connecticut lab reportedly found high amounts of the cancer-causing chemical benzene were generated by some of Target's Up & Up acne treatments and other popular skin care items.
The lawsuit states that had Target said on its labels there was benzene in its benzoyl peroxide (BPO) acne products and...Read more

How Amazon became the largest private EV charging operator in the US
Amazon’s Maple Valley, Washington, warehouse is built for speed. At night, big rigs pull up to one end to unload boxes and padded mailers – some after a short drive from a bigger warehouse down the road, others following a flight in the hold of a cargo plane. Waiting employees scan, sort and load them into rolling racks.
Before 7 a.m. each...Read more
Popular Stories
- False offers of cash subsidies used to 'capture' health insurance customers, lawsuit alleges
- Small-business owners brace for uncertainty as $20 hourly fast-food wage takes effect
- Accounting firms are prioritizing work-life balance as they face a 'human capital issue' -- even during tax season
- Broken and unreliable EV chargers become a business opportunity for LA's ChargerHelp
- Dozens of Google employees protest use of company's tech for war in Gaza