Business
/ArcaMax

Biden administration adopts new rules to boost overtime pay. California was way ahead
The Biden administration on Wednesday adopted new rules that starting July 1 will enhance overtime protections for millions of U.S. white-collar workers who receive salaries but typically work in relatively low-paying jobs.
More than an estimated 3 million Americans could stand to benefit from the plan when they work more than 40 hours a week. ...Read more

Questions swirl over the future of TikTok. Who could own it? How will the platform operate?
TikTok on Wednesday faced a formidable threat to its business, with a new law signed by President Biden that could dramatically change the way the popular video app operates.
TikTok, which is owned by Chinese company ByteDance, has faced scrutiny from U.S. government officials over how it handles the data of its U.S. users and its ties to China...Read more

Legislation banning TikTok is coming this week. How will it affect the music industry?
On Tuesday, the U.S. Senate passed a bill that would force TikTok's China-based parent company to sell the popular app, or it would be blocked in the U.S.
While the legislation would have profound impacts across the tech, political, entertainment, media and marketing worlds, music may be especially affected. TikTok's first incarnation was as a ...Read more

Ford Q1 profits fall 28% as Ford Blue hit from F-150 ramp-up
Ford Motor Co. said Wednesday it made $1.3 billion in net income in the first quarter of 2024, a 28% decrease year-over-year, as earnings from Ford's gas-engine and hybrid business tumbled from the ramp-up of the refreshed 2024 F-150 pickup truck.
The profit that represented 33 cent earnings per diluted share came on $42.8 billion in revenue, ...Read more

GM's CEO no longer highest paid Detroit auto executive
General Motors Co. CEO Mary Barra is no longer the highest-paid Detroit automotive company chief executive, receiving a 2023 compensation package totaling $27.8 million — trailing Stellantis NV CEO Carlos Tavares' $39.491 million payout.
Barra, 62, saw her pay drop 4% from her 2022 compensation of $28.97 million with a decline in her bonus ...Read more

Chicago-based Dutch Farms makes bid to buy bankrupt Oberweis Dairy
Oberweis Dairy, which filed for Chapter 11 bankruptcy protection earlier this month, announced Tuesday that it has found a buyer for its century-old family business.
Brian Boomsma, owner of Chicago-based Dutch Farms, made a stalking horse bid for nearly all of the operating assets of the company, with plans to “operate and grow the business�...Read more

Why Disney is doubling down on theme parks with a $60 billion plan
Over the decades since Walt Disney opened his first theme park in 1955, the company's tourism business has ballooned to an enterprise worth tens of billions in yearly sales, with sprawling locations in Anaheim, Orlando, Paris, Shanghai, Hong Kong and Tokyo.
Today, the entertainment giant is doubling down once again. Disney plans to invest $60 ...Read more

Ohio derailment costs continue to hit Norfolk Southern's bottom line
Atlanta-based Norfolk Southern reported a precipitous decline in first quarter profit, tallying hundreds of million of dollars of expenses in the fallout of its fiery derailment of a train carrying hazardous materials in East Palestine, Ohio, more than a year ago.
The company recorded $592 million in expenses in the first quarter related to the...Read more

Airlines must now pay automatic refunds for canceled flights
Airlines will now have to provide automatic refunds to travelers if flights are canceled or significantly altered under new U.S. Department of Transportation rules, a significant change for consumers that could drive up costs across the industry.
The final regulations released Wednesday outline the circumstances where passengers are entitled to...Read more

Amazon's Prime Video and Netflix are crashing TV's ad-selling party
Over the last decade, streaming video platforms have gradually siphoned away viewers from traditional TV networks. But next month, they will be confronting the legacy outlets on their own turf — by going directly for their advertising dollars.
For the first time, Amazon Prime Video and Netflix will both be part of what has long been known as ...Read more

'Don't piss off the DOJ': Veteran Las Vegas agents react to renewed push against Realtors
Multiple veteran Las Vegas real estate agents say they are largely on board with the upheaval currently going on in their industry.
It’s time for change and more transparency in their governing bodies, they say.
A federal appeals court ruled earlier this month that the U.S. Justice Department could restart its investigation into the policies...Read more

How to avoid going broke soon after starting a business
Yasameen Sajady, CEO of St. Paul, Minnesota-based Maazah, is learning that day by day, it takes awhile for profits to roll in.
The maker of Afghani-style chutneys, aioli and lentil dips is expanding into Target, Whole Foods and Costco stores across the country this year.
While a major opportunity, Maazah has to finance the increase in ...Read more
Her sinus surgery went great -- until she got the $32,449 medical bill
Once her doctor recommended sinus surgery, and insurance confirmed prior authorization wasn't needed, Christine Knirk focused on getting the procedure and getting better.
The outpatient operation happened just over a year ago and has brought the medical relief Knirk sought.
But getting the procedure paid for by insurance has created months of ...Read more
Tesla soars as Musk's cheaper EVs calm fears over strategy
Tesla Inc. shares surged after Elon Musk vowed to launch less-expensive vehicles as soon as late this year, easing concerns about disappointing earnings results and diminished growth prospects.
The automaker said Tuesday it’s accelerating new models using aspects of a next-generation platform that had been slated for the second half of next ...Read more
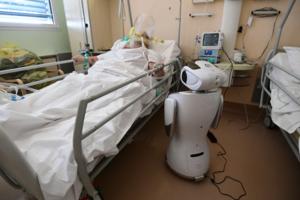
Evan Ramstad: Health care is a tough arena for AI to make a difference
It was a warp-speed tour of what's happening with artificial intelligence in medicine.
Chris Manrodt, an R&D manager for Philips' medical imaging business in Plymouth, Minnesota, last Friday gave a presentation to several hundred Twin Cities software developers and health care executives and then declared, "I feel like I've said about 50 ...Read more
Boeing retaliated against its own engineers working for FAA, union says
Boeing’s white-collar union alleged Tuesday that company management retaliated against engineers overseeing design work on behalf of the Federal Aviation Administration, heightening concerns about a self-regulation regime that’s come under renewed fire since Jan. 5, when a fuselage panel blew out midair.
In 2022, as Boeing worked to ...Read more

Google fired at least 20 additional workers after last week's Gaza protest, group says
Google fired additional workers this week, after it initially terminated 28 people who it said participated in recent protests against the company's work in Israel.
The total number of employees terminated in the wake of the protests — which took place inside Google offices in New York and Sunnyvale, Calif. — has grown to more than 50 ...Read more

UPS profit and revenue dip in first quarter. Its CEO sees growth ahead
UPS reported that its revenue and profit continued to decline in the first quarter, but its CEO sees a recovery to growth in the future.
The shipping giant reported $1.1 billion in net income for the first three months of the year, down 41% from $1.9 billion in the same period of 2023.
It had $21.7 billion in first quarter revenue, down 5.3% ...Read more

Delta is boosting pay for much of its workforce
Delta Air Lines is giving its flight attendants and ground employees a 5% raise, and boosting starting pay to at least $19 an hour for much of its workforce.
It’s the third year in a row that Atlanta-based Delta has raised pay since recovering from the worst effects of the COVID-19 pandemic. Delta granted workers 4% raises in 2022 and 5% ...Read more

PulteGroup announces 10% jump in revenues, earnings up 32%
PulteGroup last quarter had revenues of $3.8 billion, a 10% jump from the same period a year ago, as the nation’s housing shortage promises to fuel more construction, the company announced Tuesday.
The Atlanta-based homebuilding giant reported a 32% bounce in earnings, an 11% increase in the number of homes sold, as well as a higher profit ...Read more
Popular Stories
- Boeing retaliated against its own engineers working for FAA, union says
- Why Disney is doubling down on theme parks with a $60 billion plan
- 'Don't piss off the DOJ': Veteran Las Vegas agents react to renewed push against Realtors
- Airlines must now pay automatic refunds for canceled flights
- Amazon's Prime Video and Netflix are crashing TV's ad-selling party





